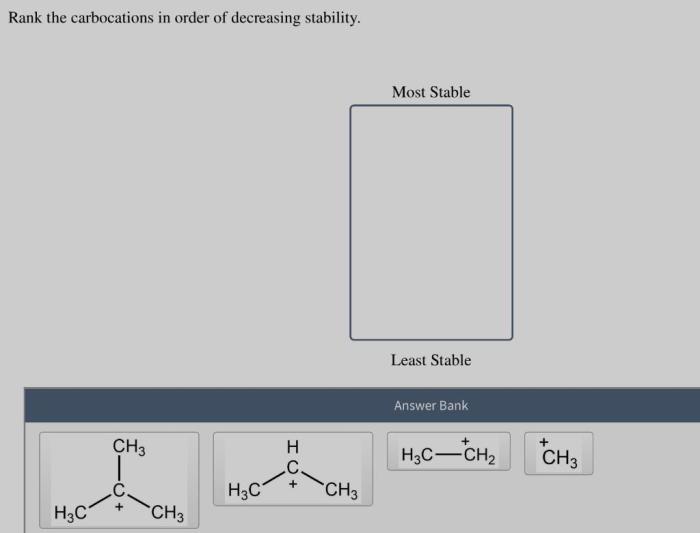
Rank the carbocations in order of decreasing stability. – Rank the carbocations in order of decreasing stability, a fundamental concept in organic chemistry, unveils the intricacies of carbocation chemistry. Carbocations, positively charged carbon ions, play a crucial role in numerous organic reactions, and their stability dictates their reactivity and reaction pathways.
Delving into the factors that influence carbocation stability, this discourse provides a comprehensive understanding of their behavior and significance in organic synthesis.
The stability of carbocations is governed by a delicate interplay of structural and electronic factors. Alkyl groups, resonance, and inductive effects collectively determine the carbocation’s ability to disperse its positive charge, thereby affecting its stability. Carbocation rearrangements, a consequence of carbocation instability, further complicate the landscape of carbocation chemistry, offering insights into the dynamic nature of these reactive intermediates.
Definition and Concept of Carbocations
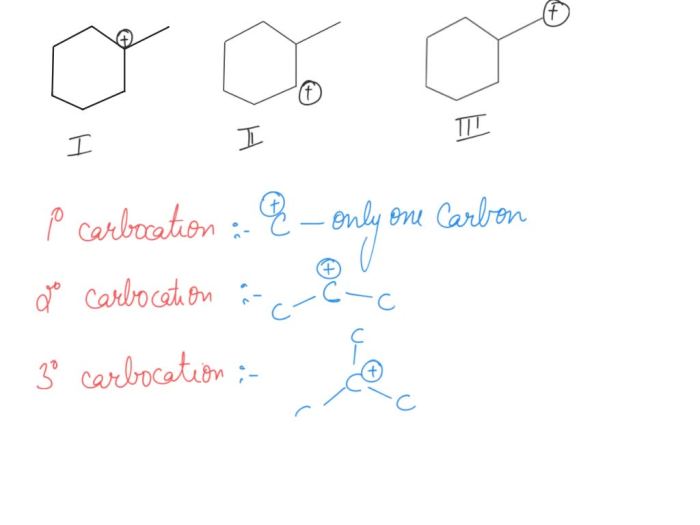
Carbocations are positively charged carbon ions that contain a vacant p-orbital. They are highly reactive and unstable, and their stability depends on several factors. Carbocations are classified as primary, secondary, or tertiary based on the number of alkyl groups attached to the positively charged carbon atom.
Primary carbocations have one alkyl group attached to the positively charged carbon, secondary carbocations have two alkyl groups, and tertiary carbocations have three alkyl groups. The stability of carbocations increases with the number of alkyl groups attached to the positively charged carbon atom.
Factors Affecting Carbocation Stability
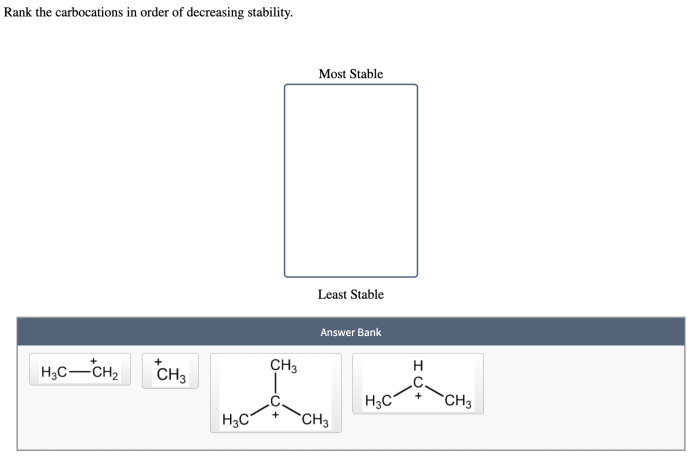
The stability of carbocations is influenced by several factors, including the number of alkyl groups attached to the positively charged carbon atom, resonance, and inductive effects.
- Alkyl groups:Alkyl groups are electron-donating groups that stabilize carbocations by donating electrons into the vacant p-orbital of the positively charged carbon atom. The more alkyl groups attached to the positively charged carbon atom, the more stable the carbocation.
- Resonance:Resonance is a phenomenon that occurs when a carbocation can be represented by two or more Lewis structures. Resonance stabilizes carbocations by distributing the positive charge over multiple atoms. The more resonance structures that can be drawn for a carbocation, the more stable the carbocation.
- Inductive effects:Inductive effects are the electron-withdrawing or electron-donating effects of substituents on a carbocation. Electron-withdrawing substituents destabilize carbocations by withdrawing electrons from the positively charged carbon atom. Electron-donating substituents stabilize carbocations by donating electrons into the positively charged carbon atom.
Carbocation Rearrangements
Carbocation rearrangements are reactions in which a carbocation rearranges to a more stable carbocation. Carbocation rearrangements can occur by a variety of mechanisms, including 1,2-shifts, 1,3-shifts, and Wagner-Meerwein rearrangements.
- 1,2-shifts:In a 1,2-shift, a hydrogen atom or an alkyl group migrates from a carbon atom adjacent to the positively charged carbon atom to the positively charged carbon atom. 1,2-shifts are the most common type of carbocation rearrangement.
- 1,3-shifts:In a 1,3-shift, a hydrogen atom or an alkyl group migrates from a carbon atom two atoms away from the positively charged carbon atom to the positively charged carbon atom. 1,3-shifts are less common than 1,2-shifts.
- Wagner-Meerwein rearrangements:In a Wagner-Meerwein rearrangement, an alkyl group migrates from a carbon atom adjacent to the positively charged carbon atom to a carbon atom that is two atoms away from the positively charged carbon atom. Wagner-Meerwein rearrangements are less common than 1,2-shifts and 1,3-shifts.
Expert Answers: Rank The Carbocations In Order Of Decreasing Stability.
What is the significance of carbocation stability in organic chemistry?
Carbocation stability dictates the reactivity and reaction pathways of carbocations, which are key intermediates in many organic reactions. Understanding carbocation stability allows chemists to predict the outcomes of reactions and design efficient synthetic strategies.
How do alkyl groups influence carbocation stability?
Alkyl groups stabilize carbocations by donating electrons through hyperconjugation, dispersing the positive charge over a larger molecular framework.
What is the role of resonance in carbocation stability?
Resonance delocalizes the positive charge over multiple atoms or functional groups, enhancing carbocation stability by distributing the charge more effectively.
Can carbocations undergo rearrangements?
Yes, carbocations can rearrange to form more stable carbocations through 1,2-shifts, 1,3-shifts, or Wagner-Meerwein rearrangements, which involve the migration of alkyl or aryl groups or hydrogen atoms.