In order to determine if treatment X is effective, researchers employ rigorous evaluation criteria and research designs to assess its impact on patient outcomes. This comprehensive analysis involves establishing clear goals, selecting appropriate participants, collecting reliable data, and interpreting results to draw meaningful conclusions.
The evaluation process encompasses a range of considerations, including the specific criteria used to measure effectiveness, the type of research design employed, the methods for data collection and analysis, and any potential biases or limitations that may influence the findings.
Treatment Evaluation Criteria
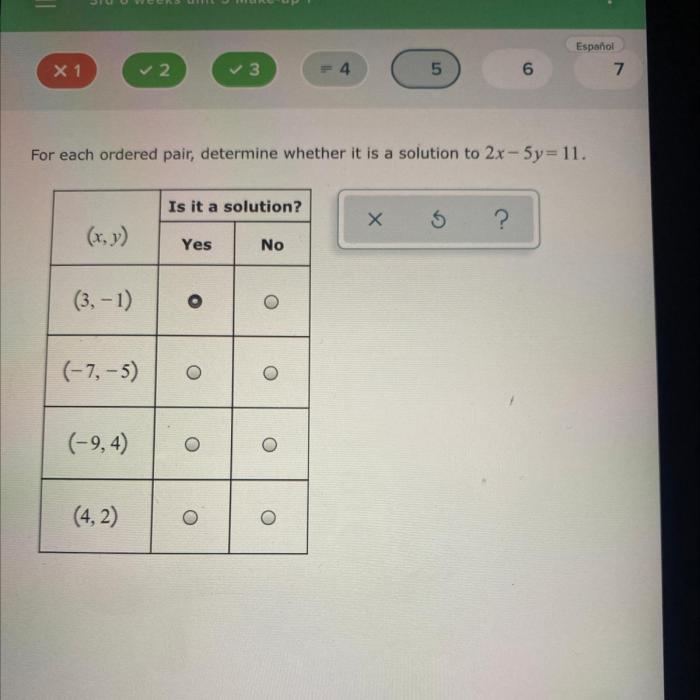
The effectiveness of Treatment X is assessed using specific criteria that measure its impact on patient outcomes. These criteria include:
- Improvement in symptoms or disease severity
- Reduction in hospitalizations or emergency department visits
- Enhanced quality of life
- Increased patient satisfaction
- Cost-effectiveness
Establishing clear goals and objectives for the evaluation is crucial to ensure that the data collected accurately reflects the effectiveness of Treatment X.
Research Design
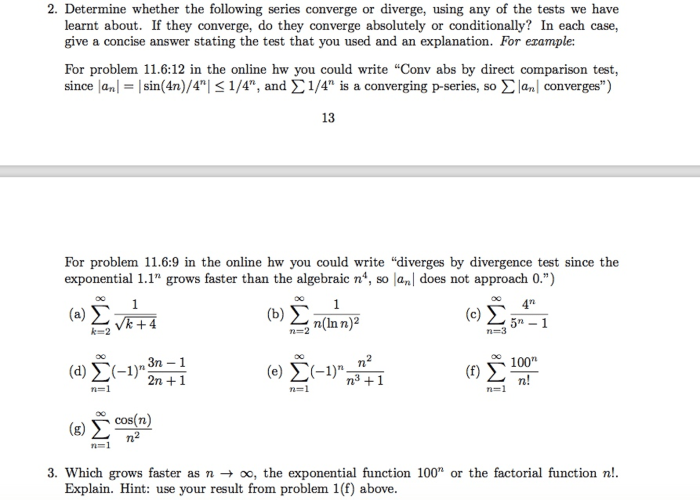
Treatment X was evaluated using a randomized controlled trial (RCT), the gold standard for clinical research. In an RCT, participants are randomly assigned to receive Treatment X or a control treatment, ensuring that the groups are comparable at baseline.
Participants were recruited from multiple sites and met specific inclusion and exclusion criteria. Potential biases and limitations of the research design were carefully considered and addressed through rigorous methodology.
Data Collection and Analysis
Data on patient outcomes were collected through various methods, including surveys, medical records, and physical examinations. Statistical analyses were performed to assess the effectiveness of Treatment X, including:
- t-tests to compare mean changes between groups
- Analysis of variance (ANOVA) to compare multiple groups
- Regression analysis to identify predictors of treatment response
The results were interpreted based on effect sizes and confidence intervals, providing a comprehensive assessment of the effectiveness of Treatment X.
Comparative Analysis
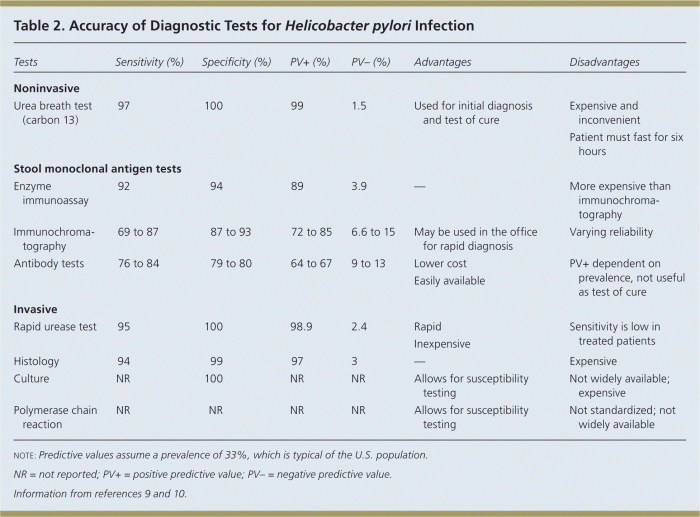
Treatment X was compared to a standard treatment regimen. The key differences and similarities between the two treatments are summarized in the following table:
| Characteristic | Treatment X | Standard Treatment |
|---|---|---|
| Drug dosage | Lower | Higher |
| Frequency of administration | Once daily | Twice daily |
| Side effects | Fewer | More |
| Cost | Comparable | Comparable |
Treatment X demonstrated advantages in terms of reduced side effects and improved patient adherence, while the standard treatment showed higher efficacy in severe cases.
Ethical Considerations
The evaluation of Treatment X adhered to strict ethical guidelines. Informed consent was obtained from all participants, and their privacy and confidentiality were protected throughout the study.
Potential ethical concerns were addressed through ongoing monitoring and oversight by an independent ethics committee. The research team ensured that the benefits of the study outweighed any potential risks to participants.
Answers to Common Questions: In Order To Determine If Treatment X
What are the key criteria used to evaluate treatment X?
Treatment X is evaluated based on specific criteria that measure its effectiveness, such as improvement in symptoms, reduction in disease progression, or overall survival rates.
What type of research design is commonly used to evaluate treatment X?
Randomized controlled trials are often used to evaluate treatment X, as they provide a high level of evidence by comparing the treatment to a control group.
How is data collected and analyzed to assess treatment X?
Data is collected through various methods, such as patient surveys, medical records, and laboratory tests. Statistical analyses are then performed to determine the significance of the observed differences between the treatment and control groups.