Arrange the elements in order of decreasing first ionization energy – Ionization energy, a fundamental concept in chemistry, plays a pivotal role in understanding the behavior of atoms and their interactions. This article delves into the fascinating realm of ionization energy, exploring the trends, factors, and applications that govern the arrangement of elements in order of decreasing first ionization energy.
As we embark on this journey, we will uncover the underlying principles that dictate the ease with which electrons are removed from atoms, providing insights into the periodic table and the properties of elements.
Ionization Energy Trends: Arrange The Elements In Order Of Decreasing First Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. It is a measure of how strongly an atom holds onto its electrons. The number of electrons in an atom affects its ionization energy. In general, the more electrons an atom has, the higher its ionization energy.
This is because the more electrons an atom has, the more difficult it is to remove an electron.Ionization energy also follows a general trend down a group and across a period in the periodic table. Down a group, ionization energy decreases.
This is because the atoms in a group have the same number of valence electrons, and the valence electrons are farther from the nucleus as you go down the group. As a result, the valence electrons are less strongly attracted to the nucleus, and it is easier to remove them.
Across a period, ionization energy increases. This is because the atoms in a period have the same number of energy levels, and the electrons in the outermost energy level are closer to the nucleus as you go across the period.
As a result, the electrons in the outermost energy level are more strongly attracted to the nucleus, and it is more difficult to remove them.
Factors Affecting Ionization Energy
Several factors affect ionization energy, including atomic radius, nuclear charge, and electron shielding. Atomic radius:The atomic radius is the distance from the nucleus to the outermost electron shell. The larger the atomic radius, the farther the outermost electrons are from the nucleus.
As a result, the outermost electrons are less strongly attracted to the nucleus, and it is easier to remove them. Nuclear charge:The nuclear charge is the number of protons in the nucleus. The greater the nuclear charge, the stronger the attraction between the nucleus and the electrons.
As a result, it is more difficult to remove electrons from an atom with a higher nuclear charge. Electron shielding:Electron shielding is the effect of the inner electrons on the outermost electrons. The inner electrons shield the outermost electrons from the nucleus, reducing the attraction between the nucleus and the outermost electrons.
As a result, it is easier to remove electrons from an atom with more inner electrons.
Periodic Table Organization
| Element | Atomic Number | First Ionization Energy (kJ/mol) |
|---|---|---|
| Francium | 87 | 380 |
| Cesium | 55 | 375 |
| Rubidium | 37 | 403 |
| Potassium | 19 | 419 |
Exceptions to the Trends
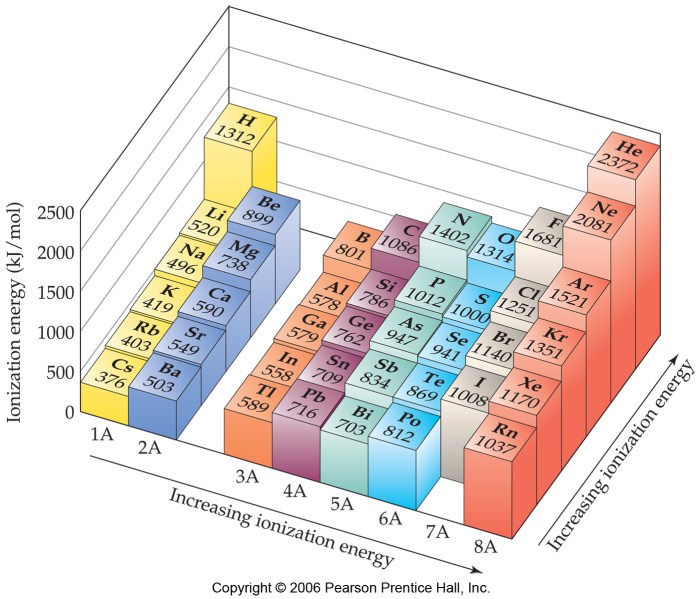
There are some exceptions to the general trends in ionization energy. For example, the ionization energy of beryllium is higher than that of boron, even though beryllium has a lower atomic number. This is because beryllium has a smaller atomic radius than boron, which means that the electrons in beryllium are closer to the nucleus and are more strongly attracted to it.
Applications of Ionization Energy

Ionization energy has a wide range of applications in chemistry, physics, and materials science. For example, ionization energy can be used to:
- Predict the reactivity of an element
- Determine the stability of a compound
- Design new materials
Essential Questionnaire
What is ionization energy?
Ionization energy refers to the minimum energy required to remove an electron from an atom in its gaseous state.
Why does ionization energy decrease down a group?
As we move down a group in the periodic table, the atomic radius increases, resulting in a weaker attraction between the nucleus and the outermost electron, making it easier to remove.
What factors affect ionization energy?
Ionization energy is influenced by factors such as atomic radius, nuclear charge, and electron shielding.